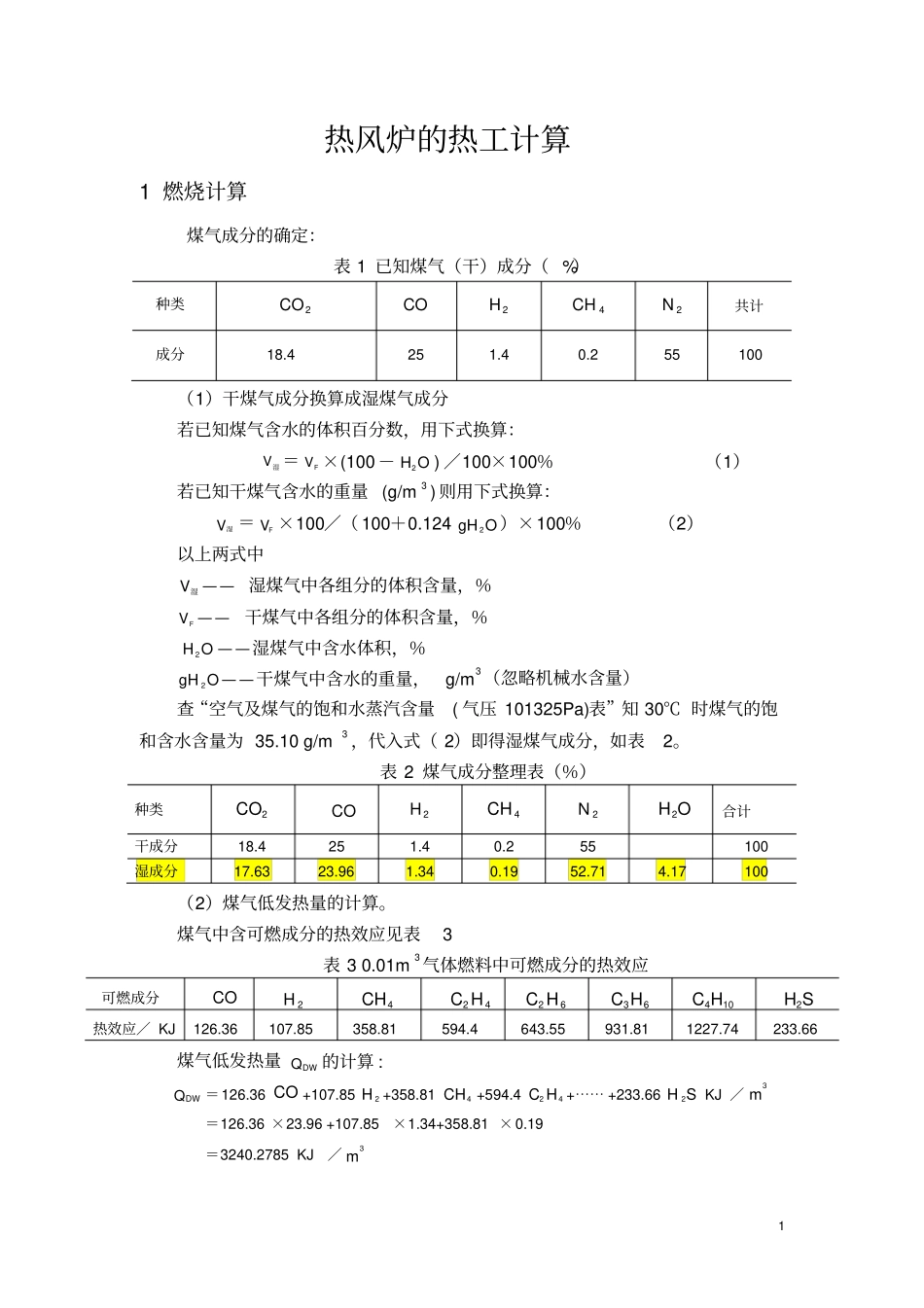
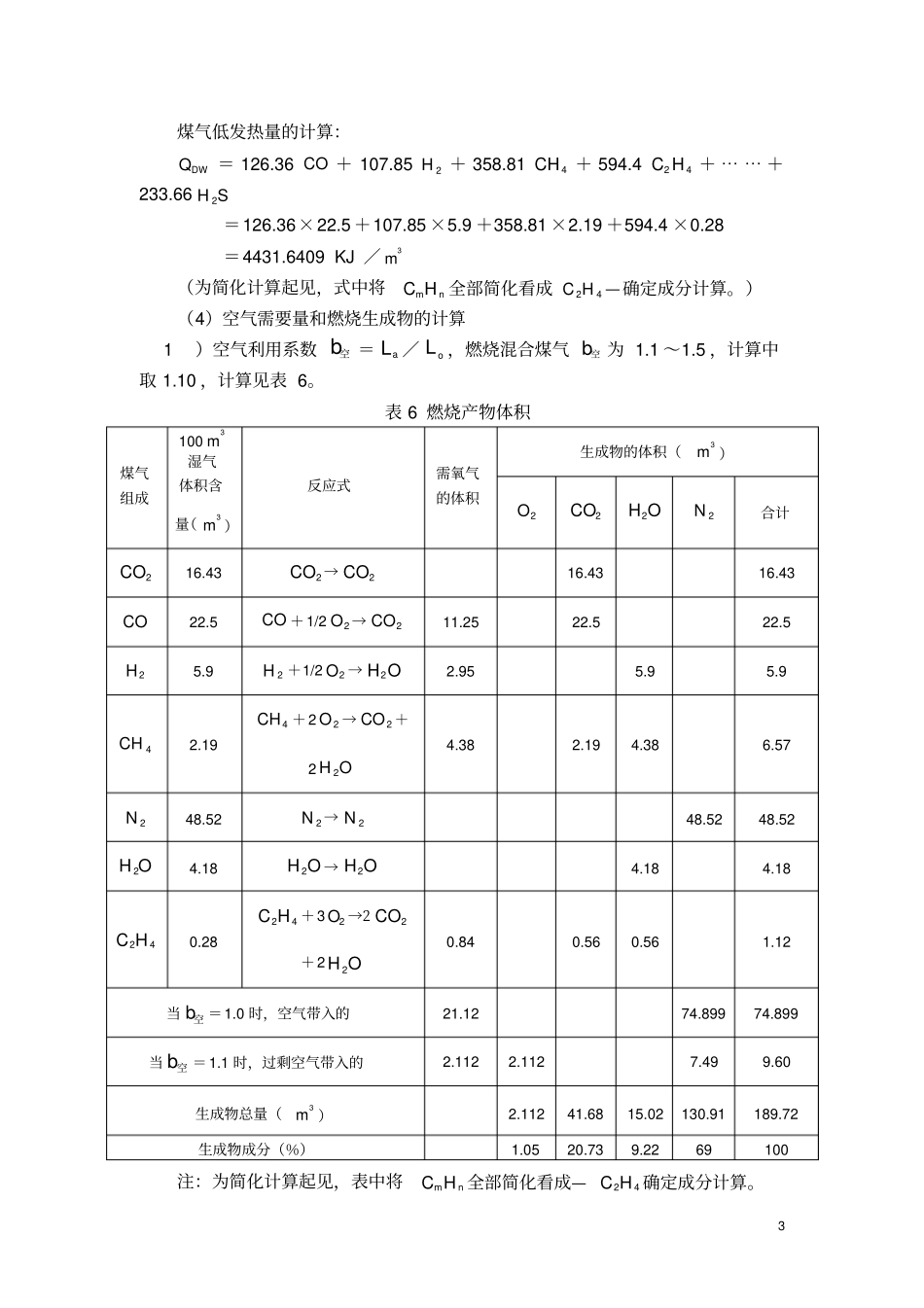
1热风炉的热工计算1燃烧计算煤气成分的确定:表1已知煤气(干)成分(%)种类2COCO2H4CH2N共计成分18.4251.40.255100(1)干煤气成分换算成湿煤气成分若已知煤气含水的体积百分数,用下式换算:V湿=FV×(100-2HO)/100×100%(1)若已知干煤气含水的重量(g/m3)则用下式换算:V湿=FV×100/(100+0.1242gHO)×100%(2)以上两式中V湿——湿煤气中各组分的体积含量,%FV——干煤气中各组分的体积含量,%2HO——湿煤气中含水体积,%2gHO——干煤气中含水的重量,g/m3(忽略机械水含量)查“空气及煤气的饱和水蒸汽含量(气压101325Pa)表”知30℃时煤气的饱和含水含量为35.10g/m3,代入式(2)即得湿煤气成分,如表2。表2煤气成分整理表(%)种类2COCO2H4CH2N2HO合计干成分18.4251.40.255100湿成分17.6323.961.340.1952.714.17100(2)煤气低发热量的计算。煤气中含可燃成分的热效应见表3表30.01m3气体燃料中可燃成分的热效应可燃成分CO2H4CH24CH26CH36CH410CH2HS热效应/KJ126.36107.85358.81594.4643.55931.811227.74233.66煤气低发热量DWQ的计算:DWQ=126.36CO+107.852H+358.814CH+594.424CH+⋯⋯+233.662HSKJ/3m=126.36×23.96+107.85×1.34+358.81×0.19=3240.2785KJ/3m2(3)焦炉煤气的加入量计算:表4焦炉煤气成分%种类2COCO2H4CHmnCH2N共计成分3.5758253.53100理论燃烧温度估算:取炉顶温度比热风温度高200℃,燃烧温度比拱顶温度约高80℃。则理T=理T+200℃+80℃=1480℃所要求的最低发热值:据经验公式:理T=0.158Q低+770Q低=(理T-770)/0.158=4494KJ/3m加入焦炉煤气量:(Q焦大约为17000~18500KJ/3m)Q焦=126.36CO+107.852H+358.814CH+594.424CH=126.36*7+107.85*58+358.81*25+594.4*3.5=18190.47KJ/m3V=(Q低-DWQ)/(Q焦低-DWQ)=(4494-3240.2785)/(18190.47-3240.2785)≈8.4%故煤气干成分加入量为1-8.4%=91.6%则混合煤气成分:VCO2=18.4%×91.6%+3.5%×8.4%=17.1484%VCO=25%×91.6%+7%×8.4%=23.488%VH2=1.4%×91.6%+58%×8.4%=6.1544%VCH4=0.2%×91.6%+25%×8.4%=2.2832%VN2=55%×91.6%+3%×8.4%=50.632%VCnHm=3.5%×8.4%=0.294%换算成混合湿煤气成分:V湿CO2=VFCO2×100/(100+0.1242gHO)×100%=16.43%V湿CO=VFCO×100/(100+0.1242gHO)×100%=22.51%V湿H2=VFH2×100/(100+0.1242gHO)×100%=5.9%V湿CH4=VFCH4×100/(100+0.1242gHO)×100%=2.19%V湿N2=VFN2×100/(100+0.1242gHO)×100%=48.52%V湿CnHm=VFCnHm×100/(100+0.1242gHO)×100%=0.28%表5混合煤气成分整理表(%)种类CO2COH2CH4N2CnHmH2O共计干成分17.148423.4886.15442.283250.6320.294100湿成分16.4322.55.92.1948.520.284.181003煤气低发热量的计算:DWQ=126.36CO+107.852H+358.814CH+594.424CH+⋯⋯+233.662HS=126.36×22.5+107.85×5.9+358.81×2.19+594.4×0.28=4431.6409KJ/3m(为简化计算起见,式中将mnCH全部简化看成24CH—确定成分计算。)(4)空气需要量和燃烧生成物的计算1)空气利用系数b空=aL/oL,燃烧混合煤气b空为1.1~1.5,计算中取1.10,计算见表6。表6燃烧产物体积煤气组成1003m湿气体积含量(3m)反应式需氧气的体积生成物的体积(3m)2O2CO2HO2N合计2CO16.432CO→2CO16.4316.43CO22.5CO+1/22O→2CO11.2522.522.52H5.92H+1/22O→2HO2.955.95.94CH2.194CH+22O→2CO+22HO4.382.194.386.572N48.522N→2N48.5248.522HO4.182HO→2HO4.184.1824CH0.2824CH+32O→22CO+22HO0.840.560.561.12当b空=1.0时,空气带入的21.1274.89974.899当b空=1.1时,过剩空气带入的2.1122.1127.499.60生成物总量(3m)2.11241.6815.02130.91189.72生成物成分(%)1.0520.739.2269100注:为简化计算起见,表中将mnCH全部简化看成—24CH确定成分计算。42)燃烧13m高炉煤气理论空气量oL为:oL=21.12/21=1.0063m3)实际空气需要量nL为:nL=1.10×1.006=1.1063m4)燃烧13m高炉煤气的实际生成物量V产为:V产=1.903m5)助燃空气显热Q空为:Q空=C空×t空×oL=1.302×20×...