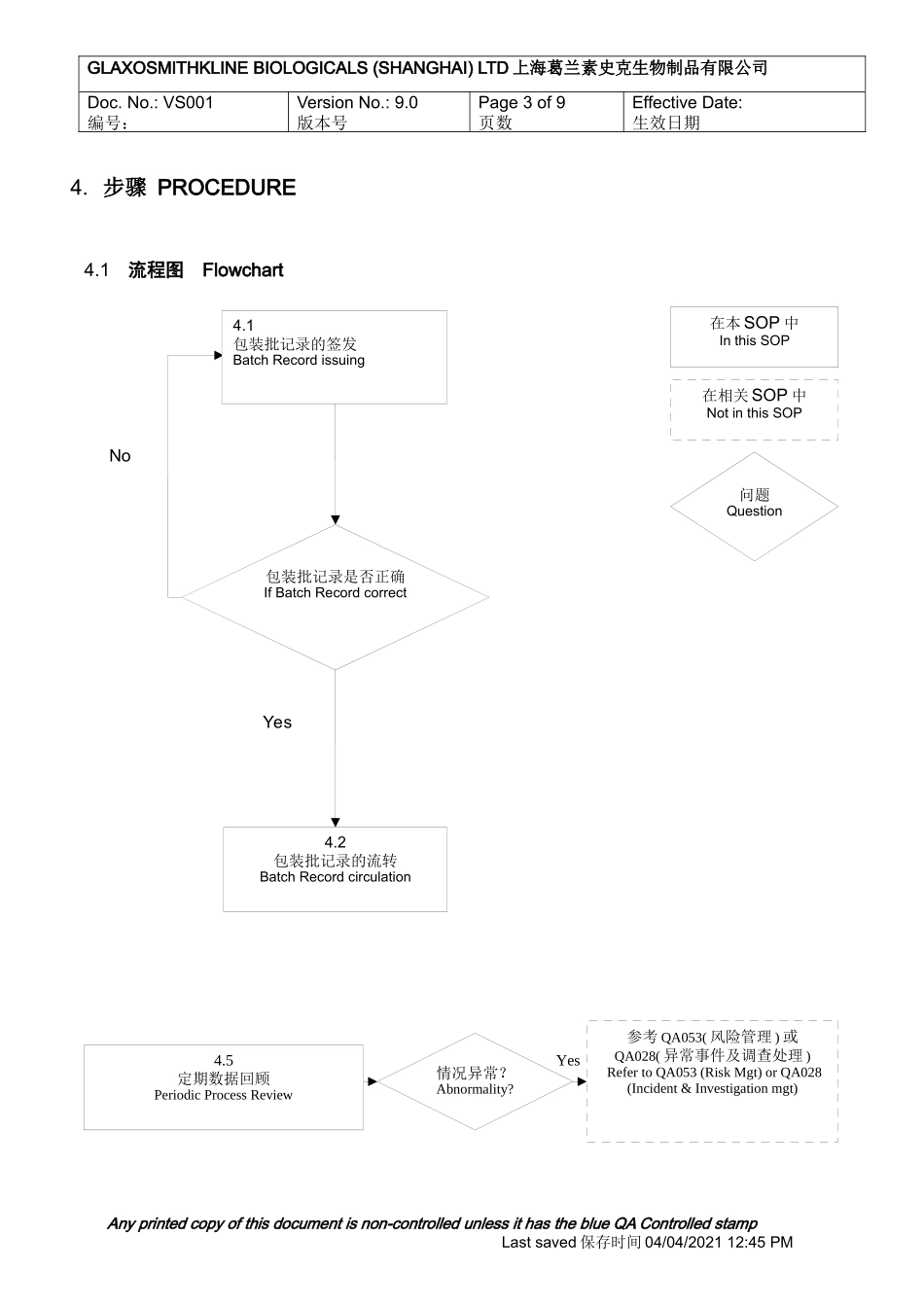
GLAXOSMITHKLINEBIOLOGICALS(SHANGHAI)LTD上海葛兰素史克生物制品有限公司Doc.No.:VS001编号:STANDARDOPERATIONPROCEDURE标准操作程序VersionNo.:9.0版本号包装批记录的签发与流转BatchRecordissuingandcirculationSupersededDate:30/10/2010替代版本日期Page1of9页数EffectiveDate:生效日期SOPinuse该流程的执行人Thefollowingpersonisaccountablefortheprocessimplementationandoutput价值流经理ValueStreamManager总页数:11页(9页+2个附件:2页)Totalpages:11pages(9pages+2appendicx:2pages)目录TABLEOFCONTENTS:1.目的OBJECTIVE.........................................................................................................................22.范围SCOPE................................................................................................................................23.职责RESPONSIBILITIES............................................................................................................24.步骤PROCEDURE......................................................................................................................34.0流程图Flowchart.............................................................................................................34.1包装批记录的签发BatchRecordissuing...........................................................................44.2包装批记录的流转BatchRecordcirculation......................................................................64.3定期数据回顾PeriodicProcessReview...........................................................................85.术语GLOSSARY.........................................................................................................................96.参考文件REFERENCES.............................................................................................................97.附件APPENDIX...........................................................................................................................98.其他相关信息RELATEDFORMSANDTRAININGMATERIAL...................................................98.1表格Forms.......................................................................................................................98.2培训资料TrainingMaterial.................................................................................................9Anyprintedcopyofthisdocumentisnon-controlledunlessithastheblueQAControlledstampLastsaved保存时间04/04/202112:45PMGLAXOSMITHKLINEBIOLOGICALS(SHANGHAI)LTD上海葛兰素史克生物制品有限公司Doc.No.:VS001编号:VersionNo.:9.0版本号Page2of9页数EffectiveDate:生效日期1.目的OBJECTIVE描述开始生产操作前,包装批记录签发给生产部门的流程TodescribetheprocesstoissuebatchrecordtothePackagingDepartmentforstartingpackagingoperations.2.范围SCOPE在上海葛兰素史克生物制品的所有包装操作TheprocedureisapplicabletopackagingoperationsinGSKBS3.职责RESPONSIBILITIES价值流部门负责包装批记录的准备。QA负责包装批记录的批准。包装部门负责包装批记录的归档,直至批记录完成后交给质保部。价值流部门负责整个流程的追踪。VSCisresponsibleforpreparingthebatchrecord.QAisresponsibleforapprovingthebatchrecord.PackagingisresponsibleforarchivingtheapprovedbatchrecorduntilsubmittedtoQAwithfinalizedBR.VSCisaccountableforalltheprocess.Anyprintedcopyofthisdocumentisnon-controlledunlessithastheblueQAControlledstampLastsaved保存时间04/04/202112:45PM在本SOP中InthisSOP在相关SOP中NotinthisSOP问题QuestionYes4.5定期数据回顾PeriodicProc...

 VIP
VIP VIP
VIP VIP
VIP VIP
VIP VIP
VIP VIP
VIP VIP
VIP VIP
VIP VIP
VIP VIP
VIP